Mental disorders are one of the most disabling diseases that affect large numbers of people in our society. Although we do not precisely know the basis of these disorders, we do know that neuronal plasticity during development and in adulthood plays a fundamental role. There is numerous evidence that point to neuronal plasticity phenomena as those responsible for the susceptibility to anxiety, stress, or as the basis of diseases such as schizophrenia or depression.
The objective of this laboratory is focused on highlighting plasticity regulation mechanisms at the basis of mental disorders and suggesting new molecular targets for the treatment in neuropsychiatric disorders characterized by disruption in plasticity processes during brain development periods or in the adulthood.
Some of the mechanisms studied in our laboratory are related to interneurons maturation regulated by transcriptional factors. Other projects are based in the gene expression regulation in different brain regions and how this is affecting to the phenotypical outcome.
- Development of animal models of psychiatric disorders.
- Histological studies in human and animal postmortem brains.
- Gene expression analyses.
- Viral control of gene expression. Stereotaxic brain injections.
- Behavioral tests.
- Surgery of rats and mice.
Selected Publications
González-Martínez J, Cwetsch AW, Martínez-Alonso D, López-Sainz LR, Almagro J, Melati A, Gómez J, Pérez-Martínez M, Megías D, Boskovic J, Gilabert-Juan J, Graña-Castro O, Pierani A, Behrens A, Ortega S, Malumbres M. Deficient adaptation to centrosome duplication defects in neural progenitors causes microcephaly and subcortical heterotopias. JCI Insight. 2021 Aug 23;6(16):e146364. Impact Factor: 8,315. Q1
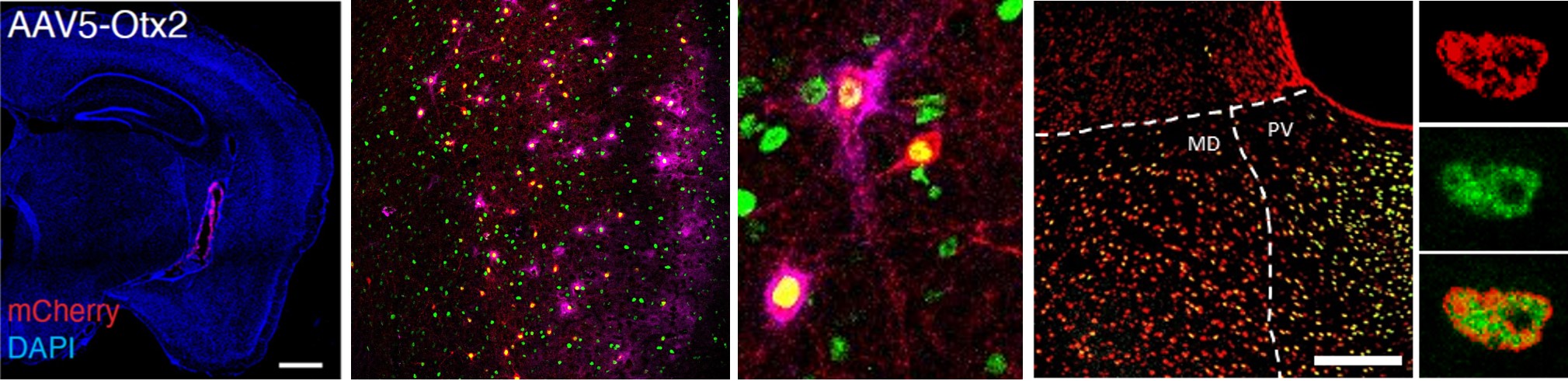
Vincent C*, Gilabert-Juan J*, Gibel-Russo R, Alvarez-Fischer D, Krebs M-O, Le Pen G, Prochiantz A, Di Nardo A A. Non-cell autonomous OTX2 transcription factor regulates anxiety-related behaviors in the mouse. Molecular Psychiatry 2021. In Press *Equal contribution. Impact Factor: 15,992. Q1
Sanjuán J, Castro-Martínez XH, García-Martí G, González-Fernández J, Sanz-Requena R, Haro JM, Meana JJ, Martí-Bonmatí L, Nacher J, Sebastiá-Ortega N, Gilabert-Juan J*, Moltó MD*. FOXP2 expression and gray matter density in the male brains of patients with schizophrenia. Brain Imaging Behav. 2020 Jul 30. *Corresponding authors. Impact Factor: 3,978. Q1
Garcia-Mompo C, Curto Y, Carceller H, Gilabert-Juan J, Rodriguez-Flores E, Guirado R, Nacher J. Δ-9-Tetrahydrocannabinol treatment during adolescence and alterations in the inhibitory networks of the adult prefrontal cortex in mice subjected to perinatal NMDA receptor antagonist injection and to postweaning social isolation. Translational Psychiatry 2020 Jun 1;10(1):177. Impact Factor: 5,280. Q1
Journiac N*, Gilabert-Juan J*, Cipriani S, Benit P, Liu X, Jacquier S, Faivre V, Melinte E, Hourcade T, Delahaye-Duriez A, Csaba Z, Lebon S, Violle-Poirsier C, Coulpier F, J-F Oury, Adle- Biassette H, Wang Z-Q, Manil S, Rustin P, Gressens P, Nardelli J. Cell metabolic alterations due to Mcph1 mutation in microcephaly. Cell Reports 2020 Apr 14;31(2):107506. *Equal contribution. Impact Factor: 9,423. Q1
Gilabert-Juan J*, López-Campos G, Sebastiá-Ortega N, Guara-Ciurana S,Ruso-Julve F, Prieto C, Crespo-Facorro B, Sanjuán J, Moltó MD. Time dependent expression of the blood biomarkers EIF2D and TOX in patients with schizophrenia. Brain Behavior and Immunity 2019 Aug; 80:909-915. *Corresponding author. Impact Factor: 6,17. Q1